Abstract
We are developing the novel αIIbβ3 pure antagonist, RUC-4, for first point of contact treatment of ST Segment Elevated Myocardial Infarction (STEMI) in combination with aspirin. We have chosen the subcutaneous (SC) route to facilitate its delivery by ambulance and emergency room personnel, and potentially by self-administration. In preparation for initiating human studies, we have conducted preclinical studies of: 1. the pharmacokinetics (PK) and pharmacodynamics (PD) of RUC-4 succinate administered IV, IM, and SC to non-human primates (NHP); 2. the impact of in vitro aspirin on the RUC-4 IC50 in human platelet-rich plasma (PRP); and 3. the effect of different anticoagulants on the IC50 of RUC-4 in human PRP.
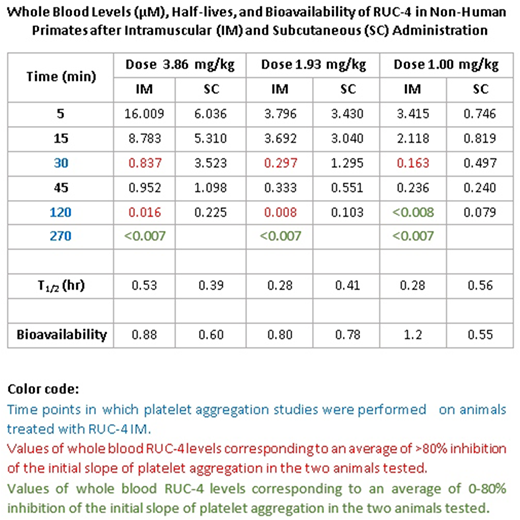
Non-human primates (Macaca fascicularis; NHPs) received RUC-4 at 1.0, 1.96, and 3.86 mg/kg IV, IM, and SC. Blood samples for IV PK studies were collected at 0, 1, 5, 15, 30, 120, and 270 min; samples for IM and SC PK studies were collected at 0, 5, 15, 30, 45, 120, and 270 min and ~27 hrs, the latter only for animals that received 3.86 mg/kg IM. There were 2 animals per group and 2 groups per dose, so no more than 4-5 samples were collected from a single animal. Platelet aggregation was performed with 5 µM ADP on citrated PRP from the NHPs that received RUC-4 IM. All doses of RUC-4 administered IM and SC were well tolerated, but animals demonstrated variable temporary bruising. None of the animals developed thrombocytopenia despite receiving up to 4 doses of RUC-4 over 3 months. The PK data are shown in the Table; both IM and SC RUC-4 reached dose-dependent peak levels within 5-15 min, with T1/2 s between 0.28 and 0.56 hrs. The bioavailability of RUC-4 IM and SC ranged between 0.60-0.88 at doses of 1.93 and 3.86 mg/kg; there was greater variability in bioavailability at 1 mg/kg (1.2 IM and 0.55 SC). Platelet aggregation studies demonstrated >80% inhibition of the initial slope of aggregation in response to ADP in all samples in which the whole blood RUC-4 concentration was ≥ ~8 nM, with partial inhibition at lower concentrations.
Since aspirin is the standard-of-care for initial treatment of STEMI, we plan to administer RUC-4 + aspirin. To assess aspirin-RUC-4 interactions, we compared the RUC-4 IC50 in untreated human citrated PRP to the RUC-4 IC50 in PRP pre-treated with 0.83 mM aspirin for 10 min at 22°C. This aspirin dose completely inhibited platelet aggregation to 1.5 mM arachidonic acid in each donor, indicating inhibition of thromboxane A2 production. In studies on 5 donors using 5 μM ADP as the agonist, the RUC-4 IC50 was 40 ± 9 nM in untreated PRP and 37 ± 5 nM in aspirin-treated PRP (p=0.50).
Previous studies demonstrated that citrate anticoagulation can variably enhance the antiplatelet effect of some αIIbβ3 antagonists (Phillips et al., Circulation 96:1488, 1997: Kereiakes et al., J Thromb Thrombolysis 12:123, 2001). We therefore compared the IC50 of RUC-4 in human blood anticoagulated with citrate (0.38%), heparin (15.8 U ml), and PPACK (300 nM) when stimulated with a thrombin receptor activating peptide (T6; TRAP) at 20 µM. The resulting IC50 values were 66 ± 25, 111 ± 7, and 122 ± 17, respectively (n=4; p=0.05 for citrate vs PPACK and p=0.04 for heparin vs citrate). With 20 µM ADP activation, the IC50 values for blood anticoagulated with citrate and PPACK (100 µM) were 54 ± 13 and 102 ± 12 nM (p=0.001).
In summary: 1. In accord with our studies with RUC-4 free base (Li et al., ATVB 34: 2321, 2014), IM RUC-4 succinate achieves peak blood levels in NHP within 5 min, resulting in >80% inhibition of platelet aggregation that persists for several hrs at a dose of 3.86 mg/kg. 2. SC RUC-4 achieves peak blood levels within 5-15 min; while the peak concentrations are lower than those with IM RUC-4 at the same doses, the blood levels are more sustained, with levels equivalent to the IM values producing ≥80% inhibition of initial slope persisting for several hrs. 3. Aspirin at a concentration that eliminates arachidonic acid-induced platelet aggregation does not affect the IC50 for RUC-4; 4. Assaying RUC-4 IC50 in human PRP from blood anticoagulated with citrate yields a value ~50% of that obtained with PRP prepared from blood anticoagulated with agents that do not affect divalent cation levels. Thus, the values in the presence of citrate may overestimate the in vivo antiplatelet effect. We conclude that the PK and PD of SC RUC-4 are favorable for short-term, high-potency inhibition of platelet aggregation as part of first point of care treatment of STEMI.
Nedelman:Biomere: Employment, Equity Ownership. Coller:Centocor: Patents & Royalties: Abciximab; CeleCor: Consultancy, Equity Ownership; Rockefeller University: Patents & Royalties: RUC-4; Platelet Biogenesis: Consultancy; Accumetrics: Patents & Royalties: VerifyNow Assays; Scholar Rock: Consultancy, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal